The problem of increasing the incidence of acute respiratory viral infections, including COVID-19 infection during seasonal hay fever, is very relevant for world health and pharmaceuticals.
 11209
11209
It is known that infectious and non-infectious antigens can contribute to additional sensitization of the body, since a variety of agents can cause allergies: pollen and plant spores, chemicals, viruses, bacteria and their toxins, micromycetes (molds and yeasts), pet skin flakes, dust , microscopic mites. Polysensitization has many etiological factors. In some cases, an infection, especially a viral one, may be the only cause of the development of an allergic disease, acting as a “superantigen”. As an example, the development of allergic rhinitis or bronchial asthma in a child after acute respiratory viral infections, the main etiological agent of which were rhinoviruses and respiratory syncytial viruses. According to the literature data, there was a correlation between the seasonal rise in the incidence of acute respiratory viral infections and the frequency of hospitalizations due to exacerbation of bronchial asthma. Also, a relationship has been found between lethal exacerbations of asthma and respiratory viral infection [1].
Compiled data from studies conducted under the international protocols ISAAC (International Study of Asthma and Allergies in Childhood) and GA2LEN (Global Allergy and Asthma European Network) showed that the incidence of bronchial asthma in the age group of 6–7 years ranged from 11.1% – 11.6%, at the age of 13-14 years – 13.2% – 13.7% in the age category of 15-18 years, the prevalence of bronchial asthma was 19.9% and 7.2% of cases, respectively. In the United States, asthma in children accounts for 8.4% of the total number of reported cases – 24.6 million people, children aged 0-4 years accounted for 4.7%, from 5 to 14 years – 9.8% [2,3].
According to O.V. Zaitseva (2007), up to 10% of the child population in the Russian Federation suffer from bronchial asthma, including severe forms [4].
According to generally accepted views, against the background of an allergic background, ideal conditions are created for the onset of an infectious process and the attachment of a secondary infection, since allergic premorbidity gives rise to a tendency to long-term persistence of an infectious agent. An example is pollinosis, which, in the absence of adequate therapy, can lead to purulent sinusitis [1].
Epidemiological and immunopathophysiological studies show that acute respiratory viral infections are the most common cause of exacerbations of allergic pathology, expressed in the clinical manifestations of bronchial asthma, in 80–85% of cases in children and 75% in adults [5,6].
In 2008, the results of a prospective follow-up of 259 children aged 0–6 years of life (Childhood Origins of Asthma (COAST)) were published in the United States. A graphical compilation of the data obtained is presented in Fig.1.
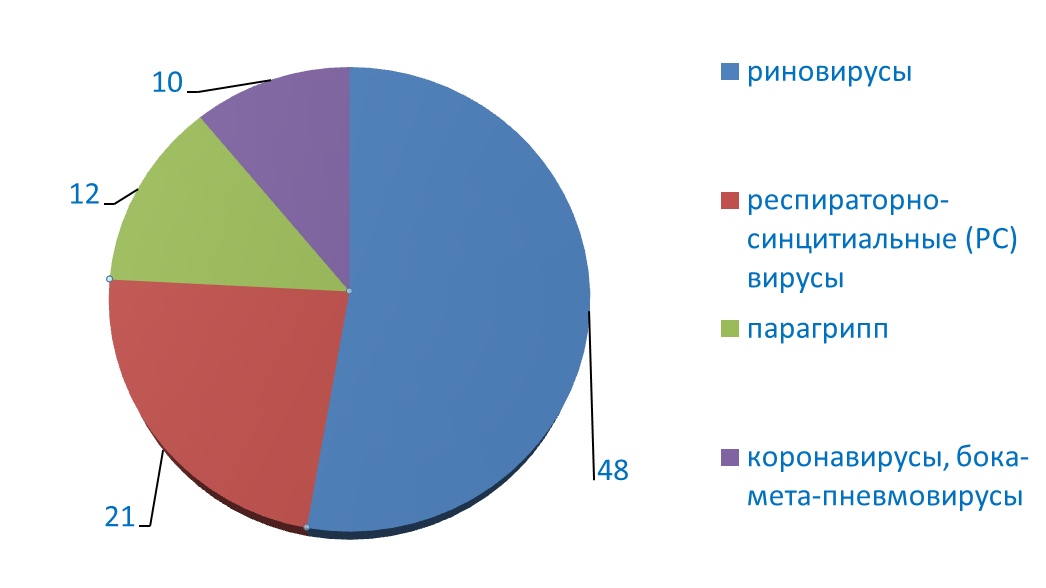 Fig. 1 COAST (Childhood Origins of Asthma) Prospective Study
Fig. 1 COAST (Childhood Origins of Asthma) Prospective Study
According to the data obtained, viruses were detected in 90% of children with wheezing under the age of 3 years (most often, rhinovirus antigens – in 48% of cases, respiratory syncytial viruses were detected in 21% of the examined, parainfluenza virus in 12% and in 10 % in patients, other types of viruses were detected – conoraviruses, boca-meta-pneumoviruses, etc. The risk of developing bronchial asthma or chronic obstructive pulmonary disease (COPD) increased if episodes of bronchial obstruction were combined with sensitization to aeroallergens. An interesting fact is that 90% of children ( 26 out of 30 examined), who had bronchial obstruction caused by rhinoviruses up to three years of age, by the age of 6 had a clear clinical diagnosis of bronchial asthma [7,8].
According to the authors, among school-age children, viral infection accounts for up to 85% of exacerbations of bronchial asthma, and viruses are more often isolated from symptomatic patients than from asymptomatic patients. At the same time, according to Satia I. et al. (2020), although the rhinovirus has been identified as the main etiological trigger for the development and exacerbations of bronchial asthma in children and adults, reliable data on the role of the new SARS-CoV-2 coronavirus in the development of bronchial obstructive syndrome and asthma is not enough today [9,10].
It should be noted that in previous outbreaks of SARS, patients with bronchial asthma, in particular children, turned out to be less susceptible to coronavirus – the authors in their 2020 work showed a low frequency of asthma exacerbations and a good prognosis during follow-up. On the contrary, according to Ludvigsson J.F. (2020), during influenza epidemics, asthma was associated with a more severe course of the disease, including the need for mechanical ventilation, not only in adults but also in children [11, 12].
During the period of increased allergic reactivity, when the release of plant pollen begins, and some animals undergo molting, releasing protein products with a high degree of sensitization into the external environment, concomitant viral infections can cause both activation and suppression of immune functions. There is a correlation between the atopic phenotype and a decrease in antiviral immunity: for example, in atopics, the synthesis of type I interferons, α and β, is impaired [13].
And the endoribonuclease enzyme of the SARS-CoV-2 coronavirus, which causes COVID-19 infection, suppresses the early activation of interferon not only in epithelial cells, but also in macrophages. It has been established that alveolar macrophages of the lung tissue, which should be the first to rush to any antigen and pathogen in the lungs, be it a virus, bacterium or chemical molecule, do not produce interferons at all in response to the introduction of the SARS-CoV-2 virus, although it is known that these macrophages actively produce interferons in any pulmonary viral infections. In addition, these macrophages do not express interferon-stimulated genes (ISG), which means that at the initial stage of infection, the immune system is not activated properly and the virus replicates in the body even before damage to the lung tissue [14-17].
So, in the process of viral phylogenesis, the new coronavirus has learned to use numerous mechanisms to suppress interferonogenesis and evade the immune response. In particular, during experiments on cell cultures, scientists found that the ORF8 protein of the SARS-CoV-2 virus directly interacts with proteins of the MHC-I major histocompatibility complex and reduces their amount on the surface of various types of cells. In cells expressing the ORF8 protein of the SARS-CoV-2 virus, MHC-I molecules are selectively directed not to the cell surface for antigen presentation, but to lysosomes, where they are degraded, as in autophagy. As a result, cytotoxic T-lymphocytes (or NK-cells – “normal killers”) cannot effectively recognize and destroy virus-infected cells [18-27].
Respiratory viral infection also acts as an independent powerful allergen in people prone to allergization. In atopic patients, an increase in the level of IgE is observed and an increase in various phases of the immune-allergic response, both immediate and delayed, is noted. Viral infection in patients with allergic diseases causes eosinophilic inflammation of the bronchial mucosa, which persists even after clinical recovery. The ability to activate eosinophils, as well as increase the level of C4 leukotriene in the nasal secretion of asthma patients, was found in rhinovirus and respiratory syncytial infection [1].
The COVID-19 infection pandemic has made its own adjustments to the behavior of allergy sufferers and atopics during the high allergic season. According to the domestic researcher Luneva E.N. (2020), patients suffering from seasonal allergic diseases, during the flowering period of plants, in the spring and summer months, it is necessary to minimize their stay in park areas, summer cottages and just on the street. Do not open windows on dry, windy days, as the pollen content in the air is especially high in such weather. After the rain, on the contrary, there is much less pollen, which means that this is the best time to ventilate the premises and walk. It is advisable to dry the washed clothes indoors, and not on the street, where the pollen of flowering plants can settle on it, which will cause allergies as soon as you put on the clothes. It is necessary to prevent the ingress of plant pollen into living quarters: close the vents and windows with a net, change clothes when returning from the street and take a shower. Additionally, you can use air purifiers. In addition, it is important to exclude from the diet foods and herbal remedies that have cross-allergenic properties with plant pollen. Under quarantine conditions, the concentration of household allergens increases. Therefore, patients who suffer from household, epidermal and fungal allergies should do wet cleaning at home more often, replace woolen blankets and down pillows with synthetic ones. It is extremely important to maintain the optimal level of room humidity in the amount of 40–50% [28].
The molecular biological features of the SARS-CoV-2 virus in terms of blocking the synthesis of interferon and the resulting interferon deficiency are evidence of the need to create high concentrations of exogenous interferon in the peripheral blood of patients to ensure adequate antiviral protection during the period of increased premorbid allergic background. The use of interferon preparations in the treatment of the infectious-allergic phenotype of bronchial asthma or COPD is widely described in the literature. So, the domestic researcher Zhakov Ya.I. et al. (2020) successfully used a preparation of recombinant interferon α2b. The authors observed a 1.6-fold decrease in IgE in sputum in children, which correlated with a decrease in eosinophils, a 1.5-fold increase in the level of IL-10, and 75% of parents expressed satisfaction with the use of the drug.
< p>According to studies conducted in the USA, today there is irrefutable evidence of the influence of interferons on the expression of genes encoding the synthesis of proteins of the incomplete, “invalid” ACE2 receptor, the so-called. deltaACE2 (dACE2). The presence of such a shortened dACE2 receptor on the membrane of epithelial cells prevents the S-protein of the SARS-CoV-2 coronavirus from connecting to it, and therefore, infection of the cell with the virus does not occur. This fact is an additional justification for the use of exogenous interferon preparations for the prevention of viral infections during a high allergophone [31].
In this regard, using our practical experience and knowledge, we would recommend the domestic preparation of recombinant interferon α2b with antioxidants – “VIFERON” as a therapeutic and prophylactic agent. The drug can be used in children from the first days of life, pregnant women, patients of various age groups. Produced for more than 20 years at a domestic GMP-certified pharmaceutical company in 3 dosage forms – gel, ointment and rectal suppositories, the drug has established itself as a reliable antiviral and immunomodulating agent that has found a worthy response in the hearts of clinicians and patients< /p>
References:
1. Tsarev S.V. Infection and Allergy: Relationship and Mutual Influence //RMJ. 2016. No 12. P. 800–803.
2. Pearce N., Aït-Khaled N., Beasley R., Mallol J., Keil U., Mitchell E. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC ).//Thorax [Internet]. 2007;62(9):758–66. Available from:
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2117323&tool=pmcentrez&re ndertype=abstract ;
3. Bronchial asthma. Clinical recommendations of the Ministry of Health of the Russian Federation, 2017;
4. Zaitseva O.V. Bronchial asthma in children //RMZH – 2007, No. 7, С.582
5.Message S., Laza-Stanca V., Mallia P. et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production //Proc Nat Acad Science 2008; vol. 105: 13562-13567;
6. Carroll K.N., Wu P., Gebretsadik T. The severity — dependent relationship of infant bronchiolitis on the and morbidity of early chldhood asthma //J Allergy Clin Immunol. 2009, May; 123(5): 1055–1061;
7. Von Linstow M., Holst K., Larsen K. et al. Acute respiratory symptoms and general illness during the first year of life: a population-based birth cohort study //Pediatr Pulmonol. 2008; 43: 584–593.
8. Macharadze D.Sh. Viruses and asthma: more questions than answers //Attending physician – 2009, No. 10, p.1-5
9. Papadopoulos N.G., Christodoulou I., Rohde G., Agache I., Almqvist C., Bruno A., et al. Viruses and bacteria in acute asthma exacerbations—a GA² LEN-DARE systematic review.//Allergy. 2011 Apr;66(4):458–68.
10.Satia I., Cusack R., Greene J.M., O'Byrne P.M., Killian K.J., Johnston N. Prevalence and contribution of respiratory viruses in the community to rates of emergency department visits and hospitalizations with respiratory tract infections, chronic obstructive pulmonary disease and asthma. PLOS One. 2020 Feb;15(2):e0228544.
11. Halpin D.M., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection?//Lancet Respir Med. 2020 May;8(5):436–8.
12. Ludwigson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. //Acta Paediatr. 2020 Jun;109(6):1088–95.
13. Gehlhar K., Bilitewski C., Reinitz-Rademacher K. et al. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients //Clin Exp Allergy. 2006 Vol. 36. R. 331–337.
14. Deng X., Aaron Vol A., , Yafang Chen Y., Kristina Kesely R.R., Matthew Hackbart M., Robert C. Mettelman R.C., et al Coronavirus Interferon Antagonists Differentially Modulate the Host Response during Replication in Macrophages//https://www.biorxiv.org/content/10.1101/782409v1.full Posted September 25, 2019).
15.Chen C., Zhou Y., Wang D.W. SARS-CoV-2: a potential noveletiology of fulminant myocarditis.//Herz. 2020;10.1007/s00059-020-04909-z. doi:10.1007/s00059-020-04909-z
16. Chiodo F. et al. //Novel ACE2-independent carbohydrate-binding of SARS-CoV-2 spike protein to host lectins and lung microbiota. //BioRxiv, May 14, 2020; DOI: 10.1101/2020.05.13.092478 )
17. Dalskov L., et al. //SARS‐CoV‐2 evades immune detection in alveolar macrophages. //EMBO Reports (2020)e51252; DOI: 10.15252/embr.202051252
18. Smirnov V.S., Totolyan A.A. Innate immunity in coronavirus infection. //Infection and immunity. 2020, Volume 10, No. 2; https://doi.org/10.15789/2220-7619-III-1440;
19. Kikkert M. Innate Immune Evasion by Human Respiratory RNA Viruses. J Innate Immun. 2020;12(1):4-20. doi: 10.1159/000503030;
20. Abigail Vanderheiden, et al. //Type I and Type III IFN Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. //bioRxiv 2020.05.19.105437; DOI: 10.1101/2020.05.19.105437;
21 Yoriyuki Konno, et al. //SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is further increased by a naturally occurring elongation variant. //bioRxiv, May 12, 2020; DOI: 10.1101/2020.05.11.088179;
22. Melissa Saichi et al. //Single cell RNA sequencing of blood antigen-presenting cells in severe Covid-19 reveals multi-process defects in antiviral immunity //bioRxiv, 2020. DOI: 10.1101/2020.07.20.212837;
23. Ivashkiv L., Donlin L. Regulation of type I interferon responses. Nature reviews Immunology. 2014;14(1):36-49. doi: 10.1038/nri3581.
24. de Wit E , van Doremalen N , Falzarano D , Munster V. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-34. doi: 10.1038/nrmicro.2016.81. ;
26. Ana Dominguez Andres, et al. //SARS-CoV-2 ORF9c Is a Membrane-Associated Protein that Suppresses Antiviral Responses in Cells. //bioRxiv 2020.08.18.256776; DOI: 10.1101/2020.08.18.256776;
27. Y. Zhang, et al. //The ORF8 Protein of SARS-CoV-2 Mediates Immune Evasion through Potently Downregulating MHC-I. //bioRxiv, 2020.05.24.111823
28. Luneva E.N. How to behave with allergies during the coronavirus pandemic? //Asthma and Allergy, -2020, No. 1, P.19-20
30. Zhakov Ya.I., Minina E.E., Medvedeva L.V. Comparative assessment of inflammatory phenotypes of bronchial asthma in children during a prophylactic course of recombinant interferon alfa-2b. Questions of practical pediatrics. 2020; 15(1): 87–94. DOI: 10.20953/1817-7646-2020-1-87-94
31. Olusegun O. Onabajo A. Prokunina-Olsson L. et al. Interferons and viruses induce a novel primate-specific isoform dACE2 and not the SARS-CoV-2 receptor ACE2 //bioRxiv, 2020, doi.org/10.1101/2020.07.19.210955 Now published in Nature Genetics doi: 10.1038/s41588-020-00731 -9
For specialists
