Although vaccination and timely booster doses are still the best way to prevent infection, major US and European pharmaceutical companies have been talking about the possibility of new vaccines tailored specifically to omicron to the market for several months. Two weeks after its discovery, an ambitious deadline for the development of such vaccines was announced: 100 days. Let's figure out what the real state of things is.
 4391
4391
Background
Omicron is a relatively new variant of the coronavirus responsible for the COVID-19 pandemic. The strain was discovered in early November in South Africa. It successfully replaced the alpha and delta variants that preceded it and today remains dominant in most countries.
Its main feature is the speed of distribution. It is believed that not only acute patients can transmit the virus, but also people without symptoms, as well as those vaccinated. It is already clear that existing vaccines are not able to protect against the disease, although those vaccinated have a lower risk of developing a severe infection and complications.
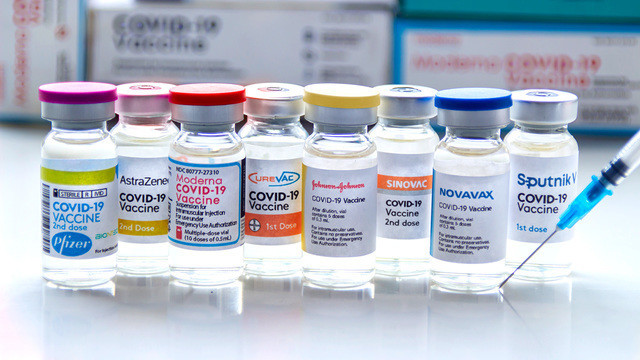
Which companies are developing a vaccine based on a new strain
Pfizer
In January, Pfizer partnered with BioNTech to begin safety clinical trials on a new vaccine that targets Omicron. It is planned to study the effectiveness of the drug both as a booster among previously vaccinated, and as a single vaccine for previously unimmunized (they will need a course of three injections). The trials will involve 1,420 volunteers aged 18 to 55, of which 205 will receive only the candidate vaccine. Preliminary data suggest that the new drug will be able to protect against Omicron as well as against other variants of the virus, and protective immunity will last longer than before.
Moderna
The American company Moderna is constantly researching new variants of coronavirus, working on obtaining drugs specific to them on the mRNA platform. A candidate mRNA-1273.529 vaccine targeting Omicron is currently in phase II clinical trials. It is supposed to be used as a booster, Spikevax, present on the market, provides reliable protection against a new version of the virus for 6 months. The company also plans to release an updated version of Spikevax (mRNA-1283) that can be refrigerated.
Novavax
Novavax's new protein vaccine NVX-CoV2373 (aka Nuvaxovid) was created based on the genetic sequence of the first variant of the SARS-CoV-2 virus discovered in Wuhan 2 years ago. Now it has already passed the initial evaluation and is currently in phase 3 clinical trials, which will have to confirm its effectiveness and safety. The studies are very extensive and include more than 30 thousand people in different countries. The vaccine has already received preliminary approval for use in the EU, New Zealand, UK, Australia, but its ability to resist Omicron remains unclear. In December 2021, the company published the first data showing a cross-vaccinal response after two doses of the vaccine, increased protection after a booster given 6 months after the main vaccination course, and high efficacy of the drug in immunizing adolescents.
AstraZeneca and Johnson & Johnson
Both Omicron and booster doses of Vaxzevria and Janssen COVID-19 Vaccine, first-generation vaccines from AstraZeneca and Johnson & Johnson respectively. As you can see, there is no need to release new versions of these drugs yet, however, representatives of both companies announced the start of work in this direction at the end of last year.
What about Sputnik V?
But the creators of the Russian vaccine Sputnik V take a clearer position. Director of the Center Gamalei of the Ministry of Health of Russia Alexander Gintsburg confidently says that the domestic vaccine does not need to be improved, and the recommendations for immunizing the population remain the same – two doses of Sputnik V and revaccination with Sputnik Light in six months.
What does this mean?
h2>
Thus, today it is impossible to say unequivocally that one of the candidates has reached the finish line of approval and commercialization of the drug. This is hindered by a number of factors. First and foremost, the virus is changing too fast, and keeping up with it is not as easy as it seems. It was no longer possible to curb Omicron, and in a few months, when at least one of the existing candidates can be on open sale, it will be possible to wait for completely new variants of the virus – everything will have to start from the beginning. In this case, the omicron can disappear at any moment, as happened with Delta. In the regions that encountered it first, new cases of infection are decreasing.
This state of affairs is forcing companies to act very carefully in order to reduce the sunk costs of developing new drugs. All the big players mentioned above say that they can only launch large trials of candidate vaccines “if necessary”…
The second reason for delays is that old vaccines still work. Numerous studies, which are constantly being updated, suggest that although the number of cases of breakthrough infection is increasing, the first generation of vaccines still resists the severe course of the disease. In addition, we must not forget that the development of a new drug and the release of billions of doses for sale are two different things. The second involves the use of industrial capacities now devoted to the production of proven vaccines, the drafting of new regulations and, finally, the readiness of states to purchase these drugs.
