The history of vaccination, antigenic variability and curious facts about infectious agents as presented by Professor of Molecular Biology Georgy Smirnov. The text will be of interest both to specialists and to those who are just starting the path of comprehending a topic that is especially acute for today, dividing society into two irreconcilable camps – supporters and opponents of vaccinations.
 24299
24299  Important!
Important!
This article contains professional specialized information, is for informational purposes only and cannot be regarded as a call for self-treatment or decision-making that is solely within the competence of a specialized specialist.
< span>Author's Preface
 Professor G.B. Smirnov
Professor G.B. Smirnov
I was forced to write this work by the events taking place in modern society and related to the pandemic. People were divided into two approximately equal camps – supporters and opponents of vaccination. In a strange way, like a pandemicCOVID-19 swept the whole world, the same world was in the power of confrontation between two irreconcilable cohorts, defending their views on vaccines. I am 78 years old. In all my life, I have never met such mass conviction and enthusiasm for my idea, which we now see in each of the opposing camps. A phenomenon surprising in its tension and mass character. I suspect that social psychologists will have something to do in the coming years. The split did not spare the families either – one of my daughters is an active and staunch character in the anti-vaxxer camp. For me, however, it still remains a mystery where the opponents of vaccination draw their conviction. After all, there is no serious scientific evidence for this. But it seems that they do not need such data – quite enough juggling, some kind of perverted fantasies. There are both honest mistakes and misconceptions. After all, both the townsfolk and pop musicians talk about vaccination and say something; people who are vague about the differences between a molecule and an atom, a virus and a bacterium, a bacterium and a unicellular protozoan.
For example, one of the blatant, grossest mistakes of opponents of vaccination is their assertion that vaccination accelerates mutations that lead to changesspike-squirrel. Their reasoning is: “The virus wants to survive, and it wants to avoid attack by antibodies.” The genome of a virus is a polymeric nucleic acid molecule, it cannot want anything, and it is not capable of striving for anything. Anthropomorphism is completely out of place here. The probability of mutation in a population is directly proportional to the size of this population. For example, in bacteria, the probability that a mutation will occur in one of the genes of one individual approaches one if the total population reaches 10⁸ – 10⁹ individuals. The same is true for viruses. That is, for the occurrence of mutations, the virus must multiply, and it reproduces very efficiently just in the cells of the unvaccinated part of the population. Vaccination dramatically reduces the likelihood of virus mutations. Of course, ignorance also plays a certain role in shaping the ideology of anti-vaxxers. Thus, by refusing to be vaccinated, a person puts himself, his loved ones, and others at risk and contributes to changes in the virus, that is, the emergence of potentially more dangerous forms of the pathogen.
I wrote the section on the mechanisms of antigenic variability in a somewhat simplified language, trying in no way to distort known scientific facts. However, this section will require from the reader, who is not familiar with the basics of molecular biology, not even tension, but simply a certain concentration. I am convinced that a person who has mastered this part will be rewarded with a sense of familiarity with the grace and exquisite beauty of the world of life.
G.B. Smirnov
Professor of Molecular Biology
Corresponding Member of the Russian Academy of Sciences
< /p>
About vaccines and vaccination
“People would rather be free ,
than reasonable”
Theodosius Dobzhansky
< span>I think his last name was Neubauer. He is disabled, a little older than me, and walks on two crutches. 1966 We are sitting on a bench next to the biological building of Moscow State University and discussing the latest work on DNA repair from damage caused by ultraviolet radiation. The latter – they are the first – this area of science is just being born. Discuss – it's not entirely accurate. He talks and I listen. I just graduated from a medical institute and came from Stavropol to Moscow specifically to get to the International Microbiological Congress. Congress President – V.D. Timakov. I already visited him about a year ago, on my last visit to Moscow. He said that I wanted to study the genetics of bacteria, showed his review on the topic “Gender and Genetics of Bacteria”, which he reported at a student circle in Stavropol. Vladimir Dmitrievich treated me favorably, asked what course I was on and, having learned that I was finishing my studies in a year, he said: “Come back in a year. You will go to graduate school with Skavronskaya. I arrived a little earlier, asked him for a guest ticket to the congress, which was immediately issued to me. I also went to Adelina Genrikhovna Skavronskaya and found out that she wanted to start working in a new direction, which she designated as “repair systems”. Meaning repair systems. What that meant, I had no idea. When I got to the congress, I did not find oral reports on this topic, but I came across the theses, where it was just about reparations. The author of the theses was a certain Neubauer. Somehow I found it. This young guy immediately realized that my knowledge in the field of DNA repair was zero and did not tell me about his experimental work, but began to enlighten me. I found out exactly which laboratories and in which countries deal with this topic, what exactly is known and what is not known at the moment. It was felt that a person is passionate about this and only lives this. And how else could he live, moving with difficulty on crutches! So I first encountered the consequences of polio. As for our conversation, it helped me a lot. In the short time before starting work in graduate school, I read quite a few articles on reparations and started working with A.G. Skavronskaya, at that time well versed in the problem.
The second time I met a polio survivor was in Germany. It was already in the mid 70s. Together with him and his girlfriend we took a road trip from Wernigerode to northern Thuringia. There they examined the memorial to Friedrich Barbarossa. The Germans had such a powerful ruler in the middle of the 12th century. Participated in 3 crusades. Our guide's name was Jörg, and he was an employee of the Wernigerode Institute. Also disabled, also on crutches. Later, Joerg Hacker moved to Würzburg, where he became the director of a major institute, and even later a member and even president of the Leopoldina, the German national academy of sciences.
Mortality in polio is not as high as in many other infectious diseases, however, its consequences are extremely serious. No one has been able to calculate how many disabled people this disease left behind in the entire history of mankind. It is also important that, striking a person in childhood, the virus leaves him crippled for life. Not escaped the sad fate and one of the best US Presidents Franklin Roosevelt. Polio vaccines – inactivated D. Salk and live A. Sabin were created in the 50s and, thanks to the efforts of M. Chumakov and A. Smorodintsev, the live vaccine has been widely used in our country and in the world since the early 60s. Two of the young scientists I met became disabled because they were born a little before the time when polio vaccine entered the children's vaccination schedule. There is now no polio in developed countries. According to official data, isolated cases of poliomyelitis are currently observed only in Afghanistan and Pakistan. Vaccination has done its job, however, analysis of very rare cases of vaccine-derived poliomyelitis in recent years has shown that work is needed to create a new modern vaccine.
Thanks to vaccines, the risk of contracting many infectious diseases has been drastically reduced or completely disappeared. And this is the most important achievement of medical microbiology. And it all began in the 18th century, at its very end. It is believed that the first person to create and apply the vaccine was the English physician Edward Jenner, and the first successfully vaccinated was an 8-year-old boy, James Phipps. It's not very accurate. The fact is that the case mentioned was the first documented, although before Jenner, other doctors vaccinated other boys and also successfully. We are talking about vaccination against smallpox. In those days, smallpox (natural or smallpox) was a very common, severe disease. Mortality in smallpox reached 30%, and in hemorrhagic form 75-100%. At the same time, the smallpox virus has a fantastic contagiousness. A person could become infected by passing by an ajar door, behind which there was a patient. There were also much more impressive examples. In the Soviet Union, very extensive studies of especially dangerous infections were carried out. Most of them were classified, many were carried out by the military. [We must immediately put an end to the and – this state of affairs was not something characteristic only of the Soviet Union. Secret bacteriological laboratories have been operating for decades and continue to operate in Fort Detrick (USA), Porton Down (Great Britain), Japan and some other countries. Moreover, after the collapse of the USSR, the United States organized such laboratories in the former Soviet republics (Kazakhstan, Georgia, Ukraine).]So, in the USSR, before the Great Patriotic War, the laboratory PNIL-52 worked on the island of Lake Seliger. At the beginning of the war, she was transferred to Kirov, then to Saratov, and in 1942 ended up on Renaissance Island in the Aral Sea. Here the laboratory was significantly expanded and turned into a testing ground called “Barkhan”. For several decades (until 1992) at the test sitebacteriological weapons were tested using experimental animals (dogs, monkeys, rats, horses, sheep). Worked with pathogens of anthrax, plague, tularemia, brucellosis and smallpox. It is with smallpox that legendary stories are associated (documents are not available for obvious reasons), about which there is information in fiction (A. Komissarenko “Plague Island”).The author writes that in 1971, a ship carrying out ecological research came close to the island, where at that time a drug based on the smallpox virus was being tested. The aerosol reached the ship and infected the people there. Returning to the city, the environmentalists infected several residents who died. According to another version of this episode, the ship was not needed – smallpox viruses traveled through the air from the island to the town of Aralsk, many residents were infected and died. So there is no doubt about the exceptional contagiousness of smallpox.
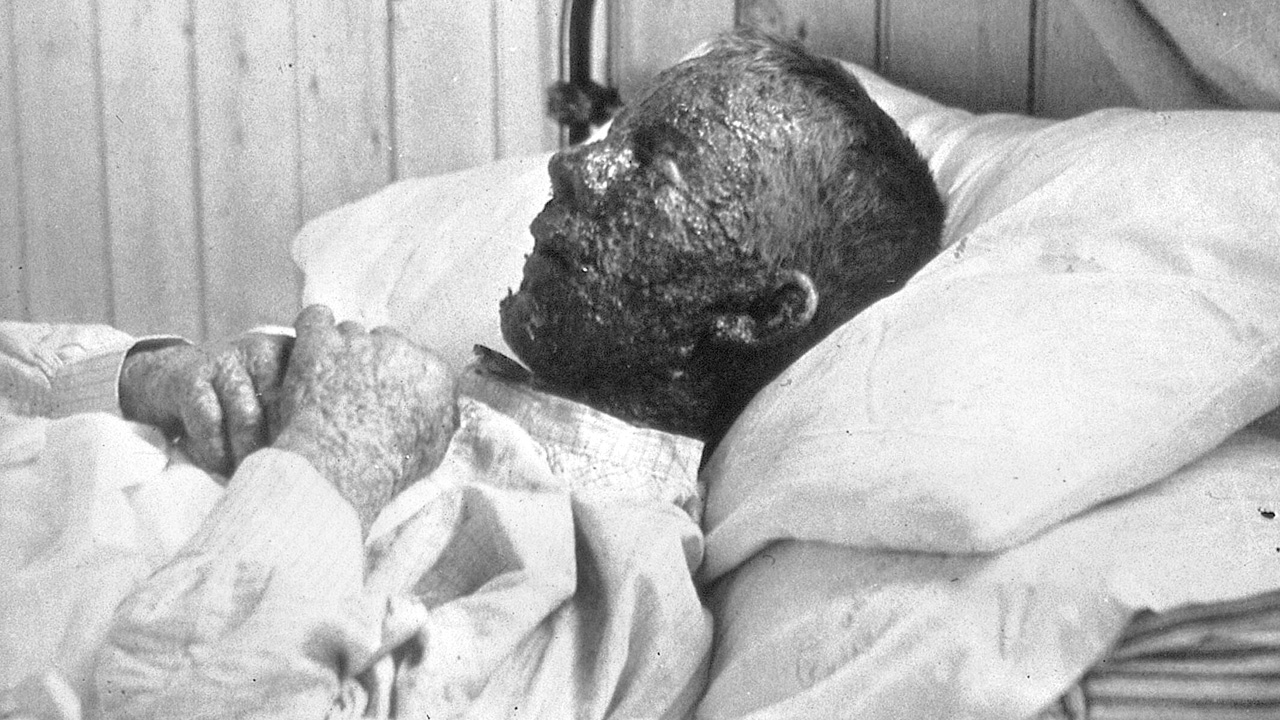 Sick with smallpox. Copyright: Wellcome Collection (Public Domain)
Sick with smallpox. Copyright: Wellcome Collection (Public Domain)
https://wellcomecollection.org/works/y2xgarem/items
The vaccination practiced by Jenner and his predecessors was based on the observation that smallpox did not affect agricultural workers (farmers) who had contact with cows with cowpox. Infection (vaccination) with vaccinia pustule material prevented smallpox disease.
Russia was also not spared by smallpox. Probably, the authorities were forced to pay serious attention to the problem when the 15-year-old Emperor Peter died of smallpox in 1730II. English specialist Thomas Dimsdale was immediately invited to Russia, who vaccinated Catherine against smallpoxII and heir to the throne, Pavel Petrovich. Mass vaccination in Russia began solemnly. In 1801, in the premises of the Imperial Orphanage in Moscow, Dr. Mukhin, in the presence of colleagues and the nobility, instilled a boy, whose name was Anton Petrov. The vaccination was successful and Anton Petrov's surname was changed. From that moment on, he became Anton Vaktsinov. I wonder if the surname has survived to this day?
The use of the smallpox vaccine around the world has been a huge success. The last natural case of smallpox infection is believed to have occurred in 1977. However, in 1978 in England, in Birmingham, a woman, medical photographer Janet Parker, who worked in the anatomy department of the Birmingham Medical School, became infected and died. Most likely, it was still a laboratory infection. In 1980, WHO solemnly declared the eradication of smallpox. The reference strains were saved. They are located in two laboratories: we have them in Koltsovo near Novosibirsk and CDC (Centers for Disease Control)— in Atlanta. It is very good that they kept it – it made it possible, when the opportunity arose, to sequence (read) the genome of the virus. Sequencing was carried out simultaneously in both laboratories – in Koltsovo and in Atlanta. It turned out that the genome of the smallpox virus consists of 200 genes. This is a lot, atypically a lot for a virus, but is consistent with the fact that the genome of this virus, unlike other DNA-containing viruses, replicates in the cytoplasm, independent of the cell nucleus. That is, the genome encodes all the proteins necessary for its own replication and particle assembly. At present, the functions of far from all proteins of the smallpox virus have been studied. It is obvious that this must be done, in particular, in order to design a modern smallpox vaccine, taking care of the future. After all, this virus, not fully understood, over the past 100 years before the elimination of the disease, according to various estimates, claimed from 300 to 500 million lives – more than all wars combined, and the threat of new smallpox epidemics should by no means be neglected.
The great Frenchman Louis Pasteur made a huge contribution to vaccinology (as well as to other branches of microbiology).
 Louis Pasteur. Copyright: Louis Pasteur. Photo: Wellcome Collection (Public Domain) https://wellcomecollection.org/works/wmzg654t/images?id=h833bwar
Louis Pasteur. Copyright: Louis Pasteur. Photo: Wellcome Collection (Public Domain) https://wellcomecollection.org/works/wmzg654t/images?id=h833bwar
It is assumed that the impetus for starting his microbiological research was the death of his three daughters from typhoid fever. Working with the causative agents of chicken cholera, Pasteur found that the old weakened microbial culture did not cause severe illness in chickens. Moreover, if chicks previously treated with the old culture were infected with the fresh culture, they did not get sick or die. In memory of Jenner and in his honor, Pasteur named the vaccination procedure vaccination (from the Latin vacca – cow). We remember that Jenner vaccinated people with cowpox. So the term “vaccine” was coined by Louis Pasteur. His next achievement was the development of vaccines against rabies and anthrax.
The causative agent of rabies is an RNA virus. Most often, a person becomes infected after being bitten by a rabid animal. In 1880, Pasteur observed the death of a 5-year-old girl bitten by a rabid dog. The impressions he received made him seriously work on anti-rabies (rabies – rabies) vaccine. It must be remembered that at that time no one knew anything about viruses, they could neither be seen nor cultivated. It is hard to imagine exactly how Pasteur thought when he planned the experiments to create this vaccine. He had only one opportunity – to use his own experience with chicken cholera, and he took advantage of this experience. Apparently, based on the symptoms of rabies, Pasteur suggested that the disease affects the brain. Therefore, he successively passaged the tissues of the brain of a sick rabbit, infecting healthy animals with them. At a certain stage, the brain tissue of a sick (or deceased) animal was dried for 2 weeks. This weakened the virulence of an unknown pathogen. Such material was used as a graft. First on rabbits, then on dogs. The procedure helped – vaccinated dogs bitten by rabid relatives did not even get sick. Pasteur was not a doctor and had no right to vaccinate people in any way. But when a boy was brought to him, bitten by a rabid dog, he decided to take a chance. 9-year-old Josef Meister, whose family lived in Alsace, was bitten by a rabid dog, and his father, who heard about Pasteur's experiments, insisted that his mother take the child to Paris, to the famous “doctor”. Then people already well knew that the consequences of rabies are fatal. Pasteur was vaccinated and Josef was saved. This was the first time a human was vaccinated against rabies. It was 1885. Before the discovery of viruses, there were still 7 years left. To Pasteur began a pilgrimage of people bitten by rabid animals. Not only the French came, but also residents of other countries. This well-deserved fame turned into a sad, one might say tragic side. Too many people asked for help too late – 2, 3 or more weeks after infection. During this time, the virus managed to get to the central nervous system, and it was no longer possible to help such patients. People were dying. Envious people and simply ignorant townsfolk began to call Pasteur a murderer. In 1887, at a meeting of the College of Medicine, Pasteur was accused of simply killing people with pieces of the brain of infected rabbits. The scientist had a second stroke, his arm and leg were paralyzed. In this state, Pasteur spent the rest of his life. He died on September 28, 1895.
In the 20th century, especially in its last third, thanks to the rapid development of science, the possibilities for designing vaccines have increased many times over.
To date, dozens of different vaccines have been created and are being used. 40 vaccines are produced in Russia alone.
The task of vaccination is to present a protective antigen to the immune system of a person or animal pathogen. The term protective refers to an antigen, in response to the entry of which into a macroorganism, antibodies are produced and other immune reactions occur that protect it from the fatal consequences of an infection caused by a natural pathogen. Such a protective antigen can be present in whole inactivated bacterial cells or viral particles (inactivated vaccines). Bacteria can be live, but in one way or another devoid of virulence, that is, incapable of causing disease (live vaccines). The vaccine may not contain whole cells or viruses at all, but may consist of their parts, including the desired antigen (chemical vaccines). There are vaccines that combine antigens of different pathogens (polyvalent vaccines). Finally, there are vaccines that do not even contain antigens, but genetic determinants that encode them (DNA vaccines and RNA vaccines). It would seem that the problems of vaccination have been solved, it remains only to apply the appropriate method of presenting the protective antigen to the immune system. However, it only seemed so.
It turned out that some infectious agents have a property that has been called antigenic variability. This means that they can change the composition of antigens. The pathogen enters a macroorganism whose immune system forms a response to its antigen(s), and the pathogen stops synthesizing these “exposed” antigens and begins to synthesize new ones that the immune system is not yet familiar with. The pathogen gets time to continue reproducing. The disease progresses unhindered. In different pathogens, antigenic variability is expressed to varying degrees. Sometimes only two antigens are interchanged. And in some cases, this is a complex program that operates with tens or even many hundreds of antigens. Here, no vaccine can help in principle. The art of “deception” of the immune system has been perfectly mastered by Neisseria (pathogensgonorrhea), borrelia (causative agents of relapsing and endemic relapsing fever, and Lyme disease), and some single-celled protozoans – causative agents of sleeping sickness and malaria. In this regard, withit is worth remembering that there are still no candidates for vaccines against the listed infectious agents, there is no effective vaccine against malaria, remember and think.
In addition to the repertoire of antigens, a very important factor in antigenic variability is the rate of antigenic substitutions. It should be noted that the mechanisms of such substitutions can be completely different. Thus, the genomes of some viruses consist of separate segments. A well-studied example is the RNA-containing influenza virus. Segments of the genome of this virus can replace each other during its circulation in nature (in cells), forming various combinations. This process is called reassortment. During reassortments, various combinations of antigens H (hemagglutinin) and N(neuraminidase), which are the basis for the classification of epidemic strains of the influenza virus. Although the influenza virus is considered to have a very high degree of variability, one or another epidemic variant (reassortant) usually circulates during the season. This makes it possible to prepare a vaccine that is valid for at least one season.
It is quite another matter if the antigens change during one infectious cycle. Here everything is determined by the peculiarities of the organization and structure of the genes encoding the corresponding antigens. Let us consider what mechanisms some bacteria and unicellular protozoa use to change antigens. Let's start with the causative agent of gonorrhea – gonococcus – Neisseria gonorrhoeae. The main surface antigen of Neisseria is the pilin protein. Villi (pili) determine the ability of bacteria to attach to a certain type of epithelial cells, i.e. determine the specificity of adhesion. The change in pilin genes during infection is called antigenic variations.
There are only two types of pilin genes:
< span>1. Expressed genes pilE1 and pilE2
2. Silent pilS genes present in the chromosome in large numbers. These genes lack the 5' portion encoding the amino-terminal sequence of the pilin protein.
Defective pilS genes are grouped into cassettes of 6 or more genes.
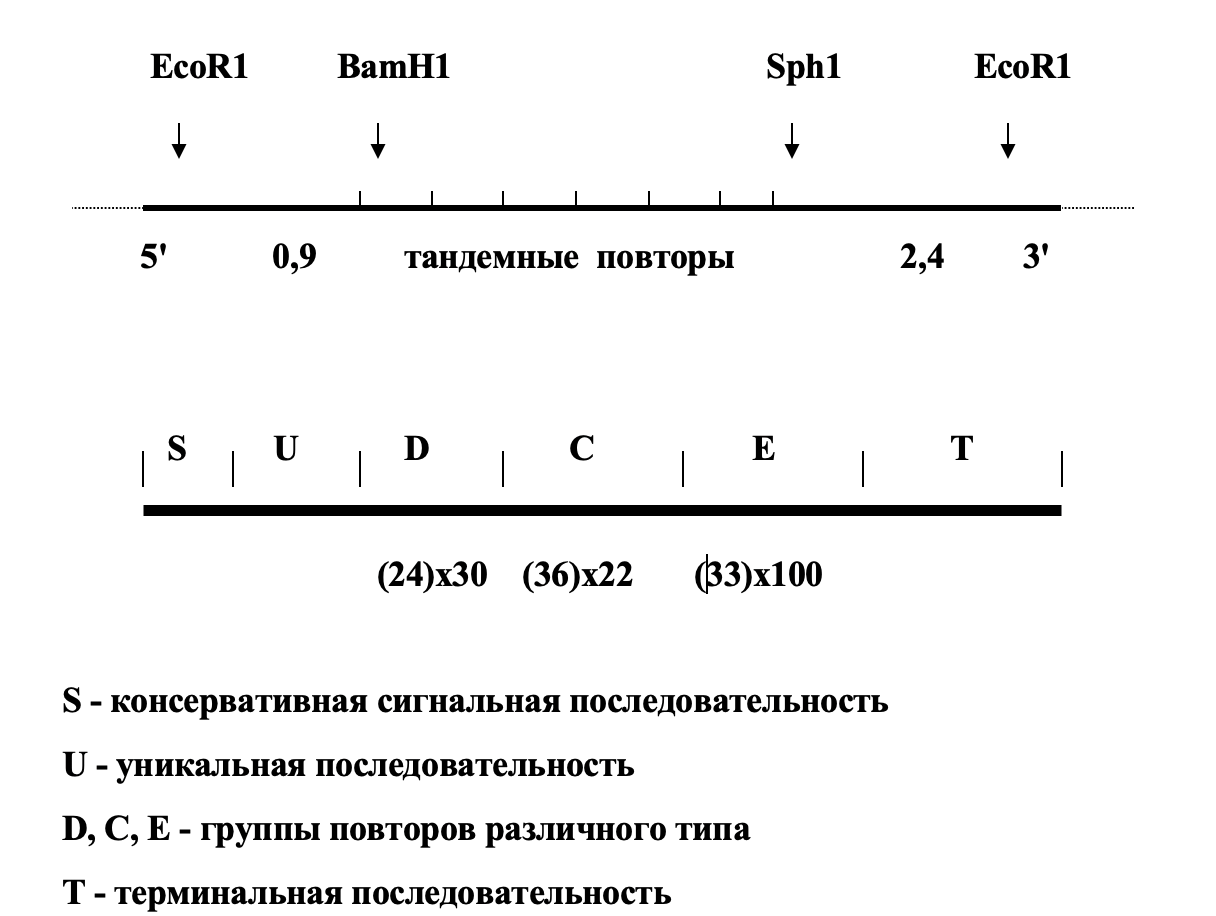
N. gonorhoeae pilin gene structure
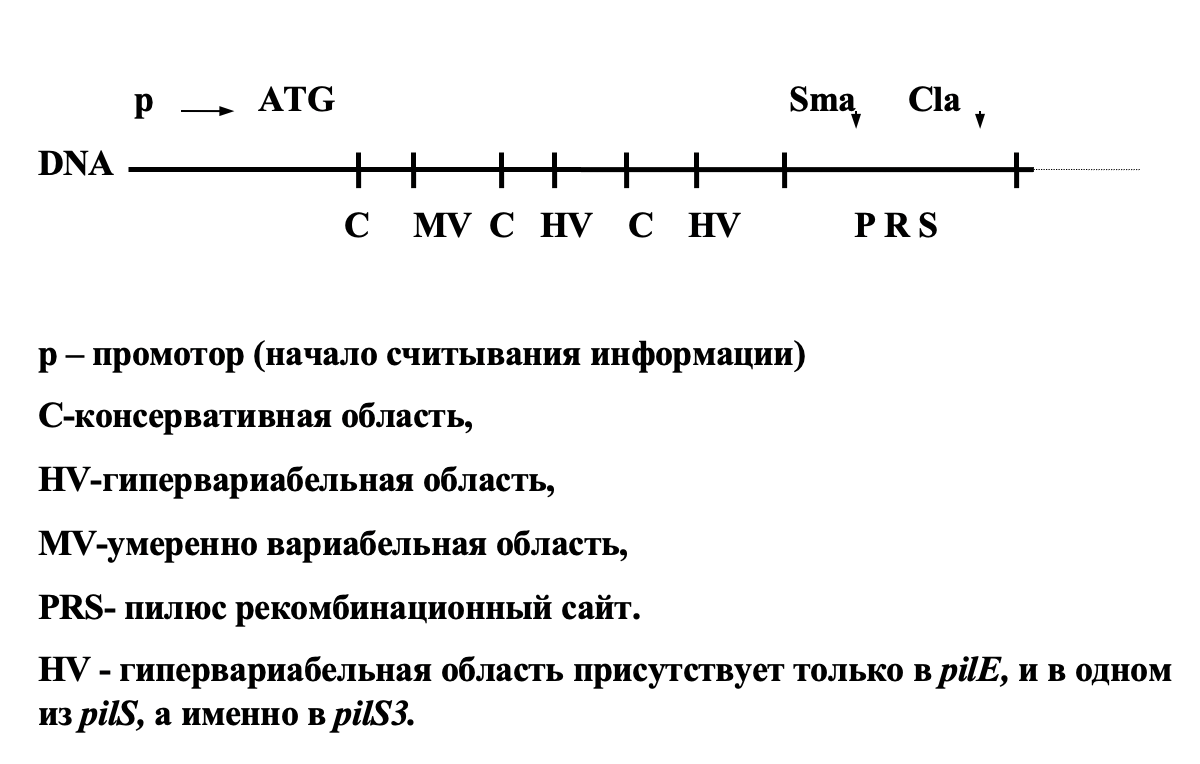
The mechanism of antigenic variation is as follows.
Mutational changes accumulate in the HV region pilS. It is important to remember that the mutation process in the pilS genes is not limited by natural selection because these genes are not expressed. Non-reciprocal RecA-dependent recombination occurs between the pilE and pilS genes, which uses the PRS site and the proximal MV region for crossing over. At the same time, only those mutations in the HV region that prevent pili assembly are rejected by natural selection. An additional source of diversity are recombination events between silent genespilS.
Pilin antigenic variations not only allow bacteria to evade immune system attacks very effectively, but also provide they have the ability to be adsorbed on various types of mucous membranes.
Clinical immunological studies are fully consistent with the concept of active antigenic variability of Neisseria, which manifests itself in the course of a particular disease. This makes understandable both the lack of immunity in gonorrhea survivors and the enormous difficulties in designing a vaccine.
Along with antigenic variations in Neisseria, there is another type of antigenic variability called phase variations . Before proceeding to the consideration of its mechanism, it is desirable to recall the features of the genetic code. The genetic code used by all living objects on Earth is four-letter and triplet, that is, it is read by certain triples of letter-symbols. Each triplet of nucleotides in a nucleic acid codes for one amino acid of a protein. In order for the text to be read correctly, reading must start from a certain point. It's called the start codon. If the start is shifted, the triplets will be incorrect and will not stand for (encode) anything. This error is called shift of the reading frame.
So, the phase variations of Neisseria affect the second surface antigen – membrane protein Opa, which is involved in the adhesion of the pathogen to the mucosa. Its presence gives the colonies turbidity, and its absence – transparency. Transitions from Ora+ to Ora- and vice versa happen at a frequency10-3in a single strain population. This is the phase variations. It turned out that there are 9 copies of genes differing from each other in the chromosome, all of them are expressed, however, only one type of protein is found in the membrane, that is, the product of only one of the 9 genes. The reason for this phenomenon is that the promoter (transcription initiation site) of the oraE gene is followed by an initiation codon and a signal sequence containing several tandem T3TT repeats. After tandem repetitions (TR–tandem repeats) is the sequence encoding the mature Ora peptide itself. The number of these TR is variable and differs in the nine available genes. To get into the correct reading frame, you must have a strictly defined number of TR (a multiple of three, for example). Only one oraE gene out of 9 has the correct reading frame in each cell. Change in the numberTRleads to the disappearance of the product that was previously present in the membrane, and the appearance of a new antigen Opa.
Scheme of the structure of the opa antigen gene
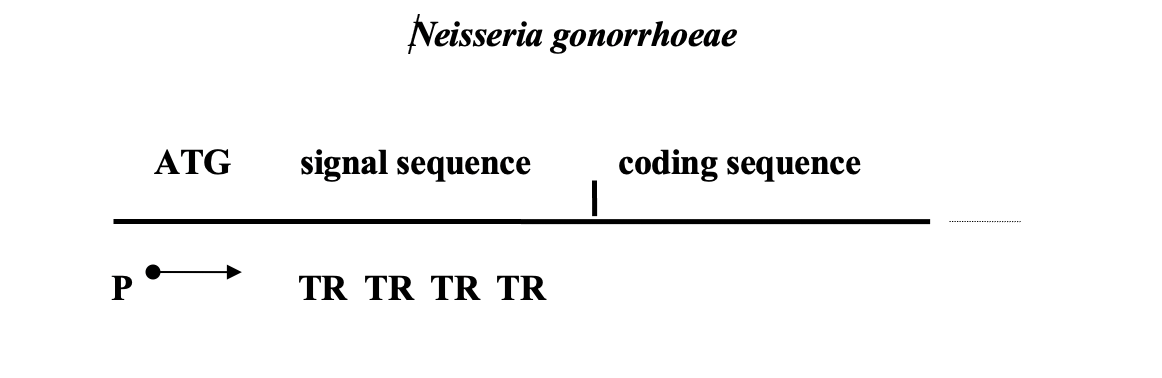
The same scheme is implemented by two other pathogens: Mycoplasma genitalium (STD agent) and Haemophilus influenza (the causative agent of meningitis and pneumonia). In the last of them, 9 genes were found containing in their proximal region short TR, represented by tetranucleotides.
Precise mechanism for quantity regulationTRyet unknown. This example of antigenic variability is not associated with rearrangements of the coding sequences of the structural genes of antigens or the movement of full-fledged genes. Instead, the number of short, non-coding repetitions located before the semantic coding sequence is changed. What factors govern this process and how they do it is completely unknown.
Let's consider what happens in the case of Borrelia.
Borrelia contain two types of plasmids: several single-copy and one multicopy (28 tnp – thousand nucleotide pairs). Single-copy plasmids contain a large number of non-expressed but otherwise complete genes encoding the structure of the VMP antigen. The multicopy plasmid has a single expressed gene vmp. Borrelia plasmids are linear. This means that one crossover is sufficient for recombination. Mutational changes accumulate in the silent genes of single-copy plasmids. Recombination between silent copies and the expressed gene results in new antigens.
Another object intensively studied in the last approximately 3 decades is the causative agent of Lyme disease– Borrelia burgdorferi, which is /span>changes its surface antigens. The plasmid of this spirochete contains one expressed (vlsЕ) and 15 “silent” copies of the cassettes with the gene of the main surface lipoproteinVLS. In genesvlsthere are 6 conservative and 6 highly variable regions; the latter probably determine the diversity of the antigenic repertoire. Both the expressed and silent copies of vlsare flanked by direct repeats of conserved 17-mer sequences TGAGGGGGCTATTAAGG. After infection, the mechanism of recombination exchange between the expressed and “silent” copies is inducedvls. 17-mer sequences are most likely recognized by recombinases that replace gene fragments vlsЕ with homologous but not identical regions of previously unexpressed copies of vls . So there is a constant change of epitopes of surface antigens of Borrelia in the body of a warm-blooded host. The genetic mechanism of the change of antigens in the causative agent of Lyme disease is very similar to that of the changePil-antigen of the causative agent of gonorrhea, and in this example there would be nothing surprising if it were not for the limitation of gene cassettes by direct repeats of 17-mer sequences. Still, as a rule, direct repeats contribute to the removal of the DNA segment enclosed between them from the genome. In this case, the opposite is true – non-expressed copies of the vls genes are retained in the plasmid genome, despite the fact that they are limited to direct repeats, that is, contrary to the law of genetics.< /p>
Tripanosomabrucei – sleeping sickness
Unlike the previously considered pathogens, these are not bacteria, but unicellular protozoa. How do trypanosomes change their antigenic composition? In trypanosomes, the genes encoding the dominant surface antigen – VSG (Variant Surface Glycoprotein) are localized in 20 telomeres (telomeres are the end sections of chromosomes, consisting of short species-specific direct tandem repeats). This antigen is present in the amount of 15 million molecules per cell. Of the 20 possible expression sites, only one works (siteELC). It expresses multiple copies of the gene encoding VSG. There are also several hundred silent copies of the VSG gene, which, accordingly, are not expressed (siteBC).
After being bitten by a tse-tse fly in the body of a vertebrate with a frequency of about 10-3VSG changes per cell per generation. The change is mediated by recombination between the expressed copy of the gene and one of many hundreds of silent homologous but not identical alleles. It is also very interesting that, according to later data, the silent copy of the VSG gene is transferred to the expression site not by itself, but as part of a group (of about 6) genes. One of the genes of this group is the transferrin protein receptor gene. This receptor provides the ability of trypanosomes to bind transferrin loaded with iron ions of a warm-blooded host. A change in receptor means the ability to bind a different transferrin and, therefore, to survive if the previous transferrin did not bring enough iron ions. The whole group of genes is transferred to the expression site with a maximum frequency of 10-3. Thus, a certain program is being implemented aimed at the appearance in the population of a fraction expressing the alternative transferrin receptor. In the event that the previous transferrin has disappeared (host change) or has become ineffective, the appearance of a new receptor will allow the fraction that has it to survive. In my opinion, what is being observed here is the implementation of a program of pre-adaptation to possible changes in the environment, rather than phenotypic selection. So, each trypanosome cell contains about 1000 unexpressed homologous VSG genes (and some transferrin receptor genes) that have not been removed as unnecessary. In fact, they are not unnecessary, as they perform a very useful function – they provide a change in antigens and transferrin receptors to avoid the action of the immune system and the possibility of utilizing iron ions when changing living conditions.
The molecular mechanism of antigenic variability in this case is not reduced to recombination, leading to the replacement of part of the gene, as is the case for pil genes in the causative agent of gonorrhea or when changing surface lipoproteinVLS in Borrelia burgdorferi, but to the transposition of full-length genes from the BC sites to the ELC site. This is a somewhat unusual transposition. In contrast to the classical one, during transposition in trypanosomes, the sequences at the ends of the translocated fragment are not always the same. Interestingly, the rate of accumulation of mutational changes in HS copies is not the same everywhere, but depends on the localization of a particular HS copy on the chromosome.
The antigen change program is also very important, that is, the sequence of translocations of BC copies, the number of of which (as we have already said) approaches 1000, and the frequency of translocations is about 10 -3. The immune system takes part in the selection of new types of antigens. This sequence is not completely random, but it is not rigid either. It is this program that leaves the host's immune system no chance of success in the fight against the pathogen. Interestingly, if you isolate the pathogen at the chronic stage of the disease and introduce it into a healthy organism, then in the new host it will start playing the program from the starting point, like a cassette rewound, while in the previous one it will continue to implement the repertoire of antigens.
Antigenic variability of the causative agent of malaria
There are several types of malarial plasmodiaPI. vivax, P.I. falciparum, PI. ovaleand PI. malariae. The most dangerous type of malaria pathogen for humans is Plasmodium falciparum. Like trypanosomes, Plasmodium are not prokaryotes, but unicellular protozoa. At different stages of infection, plasmodia multiply both in the tissues of the macroorganism (tissue cycle) and in the blood (erythrocyte cycle), using both division and sexual reproduction. Here we will not dwell on these important details.
The mechanism of antigenic variability of Plasmodium is fundamentally different from those considered earlier. There is no recombination between gene copies, no movement of homologous genes to the active site, no changes in the signal sequence. Malarial plasmodia do not have a set of homologous genes encoding surface antigens. The gene for each of the antigens is presented in a single copy. However, it is still impossible to do without repetitions. Repeats of short homologous sequences are located within the coding portion of the genes. Repeats have different lengths, from 24 to 45 bp, and are represented by different numbers of copies, from units to 100. Repeats of different lengths are partially homologous, but some groups of repeats are read in different frames. The molecular mechanisms of variability in the genes encoding Plasmodium antigens are not fully understood, but it is obvious that they are associated with transformations that depend on repeats in the central parts of the genes. Different strains and, naturally, different types of Plasmodium have different types of repeats and their number.
P. falciparum S antigen gene organization scheme
It is important to mention one more fundamental difference. New antigenic variants of Plasmodium are not antibody-selected, as is the case for trypanosomes. The change of antigens occurs in most of the population, when antibodies to the previous antigen have not yet been formed. This was also confirmed in laboratory experiments in which the selective effect of antibodies was excluded. That is, we are not talking about any natural selection in this case – antigens and the genes encoding them change in accordance with the internal rigid program. Moreover, each strain has its own antigen change program.
Thus, different pathogens use different mechanisms to create antigenic diversity and change antigens during the infectious process. This is selective expression, not accompanied by recombination within the structural gene (opa-genes of Neisseria), recombination in certain areas of structural genes, leading to the exchange of parts of genes (pilE-Neisseria genes), single crossing-over of linear Borrelia plasmids, transposition of the whole full-fledged geneVSGin trypanos. In Plasmodium, the entire mechanism of antigenic variability fits within a single gene. Programs for changing antigens are also different – from completely disorderly to absolutely rigid. It is quite obvious that whatever the molecular mechanism of antigen change, the process of antigenic variability sharply reduces the effectiveness of the immune response of the macroorganism and casts doubt on the success of the immunoprophylaxis of certain infections.
Beginning in 1975, for almost 30 years I headed one of the laboratories of the Institute of Epidemiology and Microbiology named after V.I. N.F. Gamaleya of the USSR Academy of Medical Sciences. In the 1980s, we actively cooperated with the Saratov Anti-Plague Institute “Microbe”. The subject of cooperation was the genetics of Vibrio cholerae. Research progressed well, interesting and significant results were obtained, but we were especially fascinated by the work on designing a cholera vaccine. At that time, the vaccine existed, but in no way did it correspond to the ideas about the pathogenesis of the disease and the mechanisms of immunity. As we then talked about it among ourselves – they pricked in the wrong place and in the wrong place. We were convinced that the vaccine should be live and oral. She was designed. It was obvious to us that immunity in the face of the threat of infection should be antitoxic, that is, antibodies should inactivate the cholera toxin. It was decided to design a genetically engineered vaccine. Cholera toxin consists of 2 subunits A and B. Subunit A is responsible for the toxicity of the molecule, and subunit B is responsible for immunity, that is, it is a protective antigen. Accordingly, the gene encoding the toxin also consists of two parts, which are designated ctxA and ctxB . In Vibrio cholerae, the ctxAB gene is localized in one of the chromosomes. Almost exactly the same gene (eltAB) can be part of a plasmid that is often present in E. coli bacteria (E.coli). Such E. coli is called enterotoxigenic and causes a disease that, according to the clinical picture, does not differ from cholera. We cloned the eltB gene and placed it in a vector plasmid, which was introduced into the appropriate strain E< span>. coli. After that, these same bacteria were provided with a gene that allowed them to attach to the epithelium of the human intestine. There are similar genes for adhesion to the intestinal epithelium of pigs and calves. We also worked with them. All these studies were carried out jointly with the Institute “Microbe”, where they were led by Nina Ivanovna Smirnova. The leader of our group was Vladimir Leonidovich Motin. Since the proteins of the B subunits encoded by the genes ctxB and eltB, almost identical, our potential vaccine strain perfectly protected laboratory animals from cholera, i.e. laboratory tests were very successful. Next in line were preclinical and clinical trials, which required very significant financial costs. But the 90s began and the continuation of work had to be forgotten. Vladimir Motin went to the USA, where in the laboratory of R.R. Brubaker, at the University of Michigan began work on the creation of a modern plague vaccine. He is currently a professor at the University of Texas.
A lot of time has passed since our work on the cholera vaccine.Now viral genomes are often used as vectors. There are vector vaccines for cholera A lot of time has passed since our work on the cholera vaccine. Technologies for the development of vector vaccines have advanced rapidly. Vectors were needed to deliver the desired antigen to the cells and tissues of the vaccinated organism based on adenoviruses, measles, influenza, vesicular stomatitis viruses. For example, the adenovirus platform that was used to construct the SputnikV, has been successfully used in the past to create vaccines against the Middle East Respiratory Syndrome (MERS) virus, Ebola, Lassa fever and others. So the complaints of modern opponents of vaccination that the vaccine is not tested, made hastily and “on the knee” do not correspond to reality. The main steps in the design of a vaccine are as follows. Early genes from the E1 and E3 regions are removed from the virus genome. This deprives the virus of the ability to multiply in cells. However, the ability of the genome to penetrate cells is retained. Then, in place of the E1 gene, a gene encoding the antigen against which antibodies should be developed is inserted – in the case of Sputnik V, this is the gene encoding spikespike span>protein of the coronavirus. We are getting a genetically engineered construct that can penetrate a cell, is unable to reproduce in it, but is capable of inducing the production of antibodies against the coronavirus. We see that the creation of such a vaccine required a thorough study of the nature of both adenovirus and coronavirus, the development of genome manipulation technology and the possibility of using this knowledge and experience in practice. Given the above, attempts to present SputnikV as an unfinished, dangerous vaccine are reminiscent of the arguments of the anti-vaccination opponents of Jenner or Pasteur, who called for not being vaccinated, because the procedure can turn the vaccinated into a cow.
Now, when the incredible complexity of the genetic organization of pathogens of the most dangerous infections and the sophisticated strategy of their “behavior” in an infected organism have become apparent thanks to the successes of molecular biology, one cannot but admire the courage and talent of the discoverers, who possessed very limited knowledge in this area by modern standards. Plague vaccination has a rich history. Perhaps the first experiments in this area were carried out at the end of the 18th century by the Russian scientist Danila Samoilovich Sushkovsky (Samoilovich). However, they can hardly be considered successful. Massive and quite successful experiments with the use of vaccines against cholera and plague developed by him were carried out by a student of Ilya Ilyich Mechnikov, Vladimir Aronovich Khavkin (Mordechai-Wolf Khavkin). The personality and biography of this remarkable person could well serve as material for writing a fascinating novel.
 Vladimir Khavkin. Copyright: Wellcome Collection (Public Domain) https://wellcomecollection.org/works/as9gafvt/images?id=rjtvw99x
Vladimir Khavkin. Copyright: Wellcome Collection (Public Domain) https://wellcomecollection.org/works/as9gafvt/images?id=rjtvw99x
During his stay in India, Vladimir Aronovich in 1893 tested his cholera vaccine, which at that time claimed millions of lives. The vaccine showed excellent results. However, at the very beginning of the vaccination campaign, a crowd of Indians with sticks arrived at Khavkin's laboratory with the intention of destroying the laboratory and killing the scientist. They did not understand the difference between prevention and treatment and were outraged by the fact that only healthy people receive vaccinations, and the doctor does not help the sick. The vaccine did not really help those who got sick. Khavkin went out to them, took off his shirt and injected himself with the vaccine. They did not kill. We decided to inoculate ourselves. The cholera epidemic was stopped. After 3 years, Khavkin began testing a vaccine against the plague, which then also raged in India. The vaccine was first tested on inmates at the Bikula prison. As a vaccine, he used the culture Y. pestisheat inactivated. Remarkable results have been obtained. After that, millions of people were vaccinated, and the plague receded. However, for Khavkin everything ended sadly. In 1902, several people died of tetanus. Khavkin was blamed. The scandal bore the features of anti-Semitism. His laboratory in Bombay was left without a leader. How can one not recall the accusations against Pasteur, who fought rabies. Now this is a distant story. Since then, a lot has changed. Endless experiments were carried out, new, more advanced vaccines were developed. However, in the second half of the 20th century, the Americans vaccinated their military personnel who fought in Vietnam with an anti-plague vaccine (USP), very similar to Khavkin's vaccine. The differences were that the bacteria were killed not by heating (like Khavkin), but by formalin. In our country, another anti-plague vaccine has been and is being used. This is a live vaccineEV. It is based on bacteria that have retained their viability, but have lost their virulence due to the deletion of the pgm region of the genome. The history of this vaccine is interesting in its own way. In 1926, a girl died of bubonic plague on the island of Madagascar. A virulent strain Y was isolated from her corpse. pestis,which for many years was repeatedly subcultured on artificial nutrient media. The strain has lost its virulence. In the process of studying these bacteria, it was found that the loss of virulence is associated with the deletion of a 102 kb DNA segment, which normally contains the locus responsible for hemin sorption (hms), and several genes encoding the synthesis and transport of the siderophore yersiniabactin. This deletion is not at all random and occurs at a very high frequency (approximately 10-4– 10-3per cell). Such a high frequency is due to the fact that the entire areapgmis limited by direct repetitions of the IS100 insertion element.
The information presented here about infectious agents testifies both to their differences from each other, and to the huge variety of diseases that they cause. In this regard, it is obvious that the developed vaccines should be used in different ways. Some vaccinations must be done in childhood (for example, such as against polio, hepatitis B. measles), others in case of an epidemiological situation, and others before traveling to epidemically disadvantaged areas (Africa, India).
The material of this article, of course, does not exhaust all the available data on the history and current state of vaccinology, but it gives an idea of the complexity of infectious agents and the drama of relationships in the host-parasite system. I think that this material should also inspire respect or even admiration for the memory of the ascetics who gave all their strength, and sometimes even their lives, to the cause of saving humanity from infectious diseases. Humanity is a vaccine-dependent population and, for its well-being, must continue to take care of the development of new and improvement of existing vaccines.
For specialists
