When oral bacteria combine with fungus, they form “superorganisms” that cause cavities. According to the study, these oral pests exhibit unique resistance and mobility, such multicellular symbiotic clusters, are more resistant to antimicrobials and cause deeper caries.

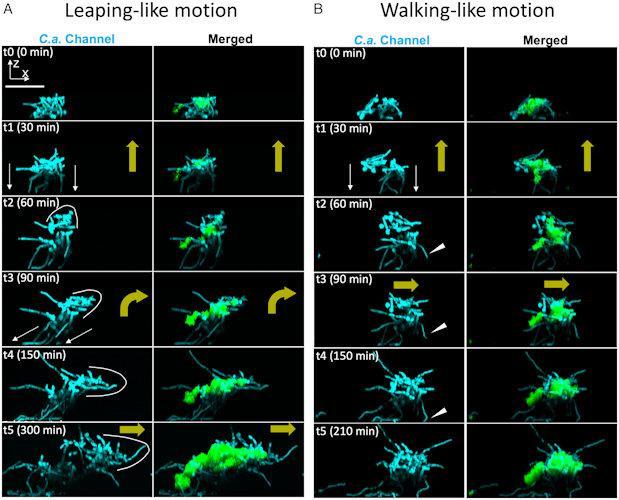
Researchers have found that when Streptococcus mutans and Candida albicans cluster together, they grow into limb-like structures, giving them unexpected superficial mobility with coordinated movements similar to “jumping” and “walking”, with continuous growth. This happens despite the fact that each embryo cannot move on its own. The researchers believe this is the first time such group-level mobility has been reported.
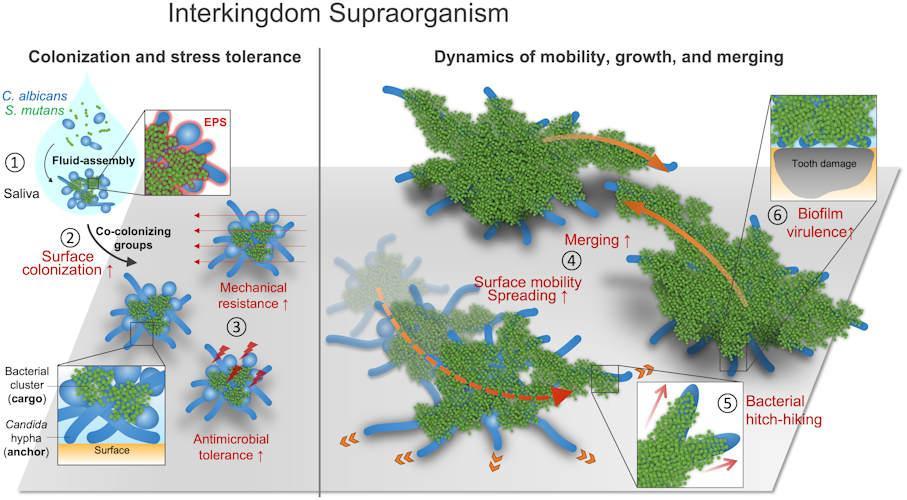
Dynamics of surface mobility and distribution at the level of the interkingdom signal chain, which is widespread among pathogenic and beneficial bacteria. All images courtesy of Ji Ren.
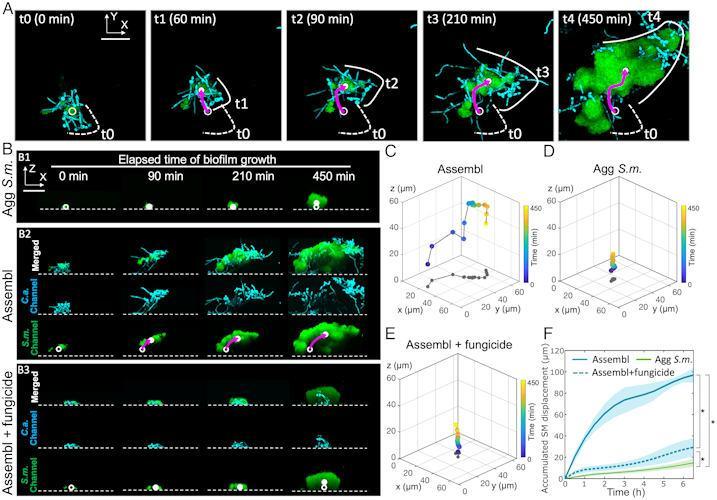
“These mobile groups of growing cells contribute to the rapid spatial spread of both species over surfaces, causing more extensive caries,” write the authors, led by Dr. Zhi Ren of the University of Pennsylvania in Philadelphia.
When fungus and bacteria combine, they form multicellular biofilms that cause many different infections in humans. However, the level at which microbes act in concert with spatial and temporal qualities to coordinate disease-promoting functions remains poorly understood.
To study the dynamics of bacterial-fungal interactions in human saliva and how they form biofilms on tooth surfaces, the researchers studied plaque and saliva samples from 14 children without caries and 30 children with deep caries. The study was funded in part by grants from the US National Institute for Dental and Craniofacial Research, the European Research Council, and the German Federal Ministry of Education and Research.
After seeing under the microscope the clumps of bacteria and fungus present in the saliva samples, the researchers conducted experiments using real-time live microscopy to observe the process of attachment and possible growth. To recreate the formation of these clusters, the authors created a laboratory system using bacteria, a fungus, and a biomaterial that resembles the structure of a tooth. They incubated microorganisms in human saliva and observed them with a microscope, which recorded their behavior in real time.
The platform allowed researchers to observe the merging of factions. They observed a highly organized structure with bacterial clusters attached to a complex network of fungal yeasts and thread-like processes called hyphae. The structure was enclosed in an extracellular polymer.
In addition, the researchers tested the properties of these clusters after they colonized the tooth surface. They found increased adhesion to the surface, which made them very sticky, as well as greater mechanical and antimicrobial resistance.
Emergent microbes
In these clusters, bacteria used fungal hyphae to attach to the surface, and then propelled the entire superorganism forward by transporting the attached bacteria across the surface of the tooth.
Jumping movement | Movement similar to walking
Community mobility, bacterial hitchhiking, and spatial growth dynamics using time series of confocal images to grow interconnected communities.
When bacteria and fungus united, they moved quickly and over quite a distance. On the tooth-like surface, the speed of microbes was more than 40 microns per hour. According to the study, this speed is similar to that of fibroblasts, which are cells involved in wound healing.
During the first hours of growth, the clusters were observed to move more than 100 µm across the surface. While many questions remain about these mechanisms, researchers know that the ability of microbial aggregations to move during growth allows them to quickly colonize and spread to new surfaces. When the clumps were allowed to attach and grow on human teeth in a laboratory model, more extensive caries was found due to the rapid spread of the biofilm.
Clusters of Interkingdom in human saliva behave like super-organisms with new functionality and disease-promoting activity.
Clinical significance
Unbelievable, but true – bacteria and fungus have gathered into a new whole, which gives each other various advantages and functions that they would not have on their own, the authors write.
“These observations may have clinical implications for the onset of severe childhood caries characterized by rapid and aggressive destruction of tooth enamel,” write Ren and colleagues.
